Popular on EntSun
- EMBER™, the Only Standardized System Linking Workforce Identity to Growth, Appoints Global Brand Visionary Bret Sanford-Chung to Board of Directors - 1073
- $3 Billion Suicidal Depression Market Advancements on Multiple Fronts, Highlighted by FDA Fast Track Designation for Effective NRX 100 Drug Therapy - 1047
- Phinge®, Home of Netverse® and Netaverse™ With Verified and Safer AI Announces "Test the Waters" Campaign for Potential Regulation A+ Offering - 1019
- The 2025 "Aizu Festival" in Aizu Wakamatsu City will be held September 19–21 - 999
- Ubleu Crypto Group Achieves FinCEN Registration and Colorado Incorporation, Accelerating U.S. Market Entry - 995
- University Rankings Index Announces 2025 Rankings of the Top US Online Universities - 966
- Perception meets learning: Museum of Illusions Orlando offers educational field trips - 949
- iPOP Alum Ava Jean lands role in the reboot of "Buffy the Vampire Slayer" - 903
- Titus Announces Triumphant Return to the Gospel Music Industry - 890
- Boston Industrial Solutions Launches Citrine® CAL-685 Silicone Primer - 819
Similar on EntSun
- Physician-Turned-Patient Launches Advocacy Campaign to Spotlight Disability Insurance Barriers
- Your Body Isn't Broken—It's Out of Balance: The New Book Revealing the Blueprint to Restore Hormone Balance, Sleep, Gut & Metabolic Health
- Youth Take the Lead: Kopp Foundation for Diabetes Hosts "By Youth, For Youth, With T1D" Gala on October 8 at Blue Bell Country Club
- New Slotozilla Project Explores What Happens When the World Goes Silent
- Counseling Center of New Smyrna Beach Expands Affordable Mental Health Services for Volusia County
- Athena Forge (ATFG) Introduces Advanced Token for Technology-Driven Financial Ecosystem
- Albuquerque's Z-CoiL Footwear Brings All-American Family Business Story to Shark Tank Season Premiere
- NoviSign Sponsoring VARTECH 2025 - the B2B IT channel's #1 event
- CCHR: For Prevention, Families Deserve Truth From NIH Study on Psychiatric Drugs
- Sheets.Market Brings Professional Financial Model Templates to Entrepreneurs and Startups
$750 Million Ketamine Drug Market Withing Reach via New Commissioner's National Priority Vouchers with Anticipated Approval by Year-End for NRx
EntSun News/11062982
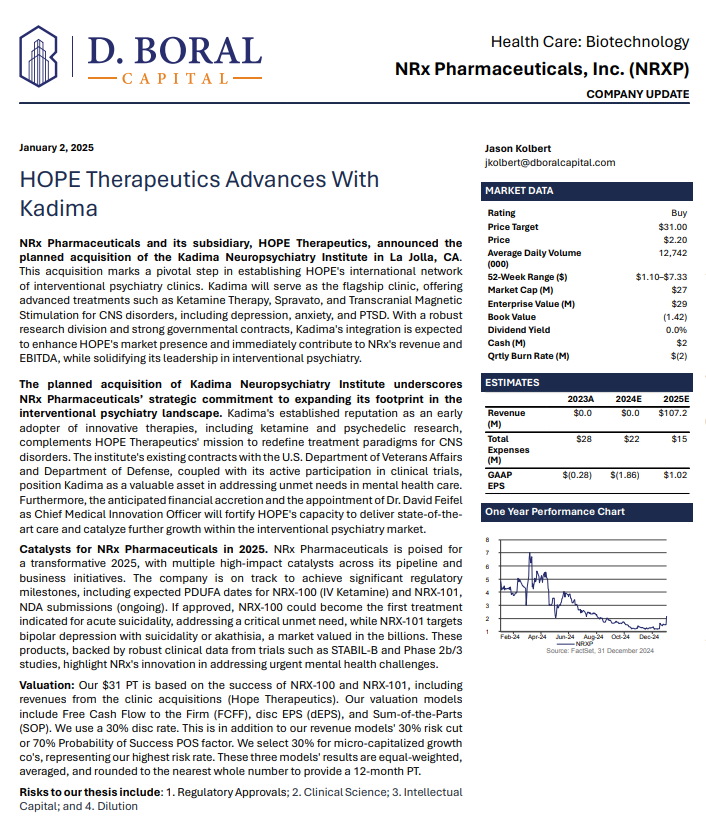
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - EntSun -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application With Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market.
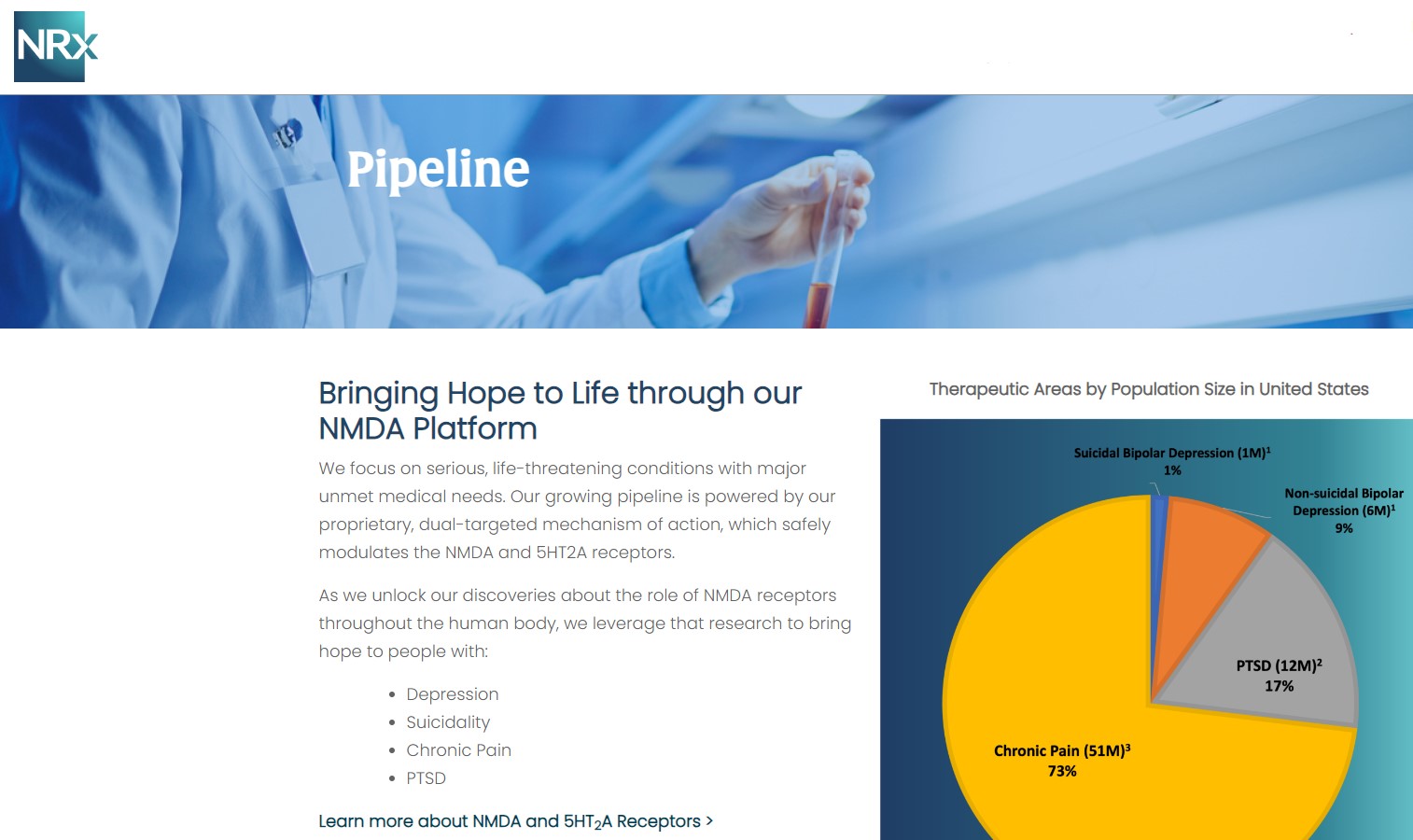
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on EntSun News
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Concurrent with the CNPV process, NRXP is preparing a citizen petition to seek withdrawal of preservative-containing forms of ketamine, based on the toxicity associated with the benzethonium chloride preservative used in the historic formulation. NRXP has also filed a patent on its preservative-free manufacturing process. Approval of either the citizen petition, or the patent, would be expected to enable NRXP to gain market share in the current $750 million generic ketamine market that is forecast to reach $3-5 billion annually by 2033, in addition to a share of the market already established for ketamine products for treating depression.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
astr partners will work with the NRXP and HOPE subsidiary executive teams to execute a comprehensive investor relations program that includes investor targeting, message development, earnings preparation, conference support, and proactive investor engagement.
Abbreviated New Drug Application (ANDA) for Preservative-Free IV Ketamine
On June 5th NRXP announced the transmission of its Abbreviated New Drug Application (ANDA) for electronic filing to the U.S. Food and Drug Administration (FDA) for NRX-100, its preservative-free IV ketamine formulation, for use in all existing approved indications such as anesthesia and pain management.
NRXP anticipates filing a citizen's petition with the FDA to remove benzethonium chloride, a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. NRXP believes that the preservative-free feature of NRX-100 will be deemed of benefit to patients because of the known toxicity of closely related benzalkonium chloride in current drug products. Preservatives were originally added to sterile injectable products in an era when a single vial of medication was used to treat multiple patients, a practice no longer allowed in US hospitals. NRXP has demonstrated that there is no need for such preservatives to maintain stability and sterility in ketamine presentations intended for single-patient use. Should the citizen's petition be granted, all formulations of ketamine sold in the US could face a regulatory requirement to be preservative free.
More on EntSun News
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application With Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on EntSun News
- Screenwriting Cruise Adds Howard Suber, Ph.D., to Inaugural 7-Day Screenwriting Lab at Sea
- Wacky Wednesday's Midweek Mayhem & Madness Ignites Delirious Comedy Club with Explosive Laughs!
- Renaissance Man: The Weekly's best comedian takes center stage
- Twice the Laughs: Comedy Star Don Barnhart Rotates Residency at Both Delirious Comedy Club Locations in Las Vegas
- Voices for Humanity Ignites a Revolution for Learning with Eva Rehorova
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Concurrent with the CNPV process, NRXP is preparing a citizen petition to seek withdrawal of preservative-containing forms of ketamine, based on the toxicity associated with the benzethonium chloride preservative used in the historic formulation. NRXP has also filed a patent on its preservative-free manufacturing process. Approval of either the citizen petition, or the patent, would be expected to enable NRXP to gain market share in the current $750 million generic ketamine market that is forecast to reach $3-5 billion annually by 2033, in addition to a share of the market already established for ketamine products for treating depression.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
astr partners will work with the NRXP and HOPE subsidiary executive teams to execute a comprehensive investor relations program that includes investor targeting, message development, earnings preparation, conference support, and proactive investor engagement.
Abbreviated New Drug Application (ANDA) for Preservative-Free IV Ketamine
On June 5th NRXP announced the transmission of its Abbreviated New Drug Application (ANDA) for electronic filing to the U.S. Food and Drug Administration (FDA) for NRX-100, its preservative-free IV ketamine formulation, for use in all existing approved indications such as anesthesia and pain management.
NRXP anticipates filing a citizen's petition with the FDA to remove benzethonium chloride, a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. NRXP believes that the preservative-free feature of NRX-100 will be deemed of benefit to patients because of the known toxicity of closely related benzalkonium chloride in current drug products. Preservatives were originally added to sterile injectable products in an era when a single vial of medication was used to treat multiple patients, a practice no longer allowed in US hospitals. NRXP has demonstrated that there is no need for such preservatives to maintain stability and sterility in ketamine presentations intended for single-patient use. Should the citizen's petition be granted, all formulations of ketamine sold in the US could face a regulatory requirement to be preservative free.
More on EntSun News
- Your Body Isn't Broken—It's Out of Balance: The New Book Revealing the Blueprint to Restore Hormone Balance, Sleep, Gut & Metabolic Health
- Youth Take the Lead: Kopp Foundation for Diabetes Hosts "By Youth, For Youth, With T1D" Gala on October 8 at Blue Bell Country Club
- Green Office Partner Named #1 Best Place to Work in Chicago by Crain's for 2025
- CCHR, a Mental Health Watchdog Organization, Hosts Weekly Events Educating Citizens on Important Mental Health Issues
- Goat Skin Chicago Partners With Inkdnylon Custom Apparel to Strengthen Brand Growth
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on EntSun News
- Basketball Meets Innovation: THE LEAGUE's Culture-Driven Platform
- Breaking: 50+ runners from 20+ states relay custom 9/11 flag 485 miles from Shanksville through DC to Ground Zero for memorial remembrance run
- SecureMaine 2025 is this October 8th in Portland, Maine
- Celebrity Psychic Jesse Bravo Brings New York's Premier Psychic to Global Audiences New York, NY
- John Thomas calls for unity and prayer after tragic loss
- From Page to Premiere: The Golden State Signature Series: A DonnaInk Publications Signature Showcase
- Where the Miami Dolphins Stand After Week 1
- Which NFL Teams Can Rebound from Week 1? OddsTrader Breaks Down the Biggest Questions
- Barry J. Neely's The Demon Detective Original Score to be Released
- iPOP Alum Pierson Fode starring in Netflix's 'The Wrong Paris"
- Genesis Creations Entertainment expanding into Soul Purpose Reports
- Apellix Deploys Breakthrough Spray-Painting Drones into Live Service Limited Beta Program Open for Advanced Contractors
- The Strand Theatre Celebrates 90 Years of Magic with a Weeklong Anniversary Celebration
- Celebrate 90 Years of The Strand: Join the $90,000 Fundraising Effort
- Brightwater Lagoon launches Football SUN-Days
- DivX Unveils New Educational Blog Series to Simplify MKV to MP4 Video Conversion
- The Gabriella Rossetti Vault Sale Is Live
- CCHR: For Prevention, Families Deserve Truth From NIH Study on Psychiatric Drugs
- Sheets.Market Brings Professional Financial Model Templates to Entrepreneurs and Startups
- Webinar Announcement: Investing in the European Defense Sector—How the New Era of Uncertainty Is Redefining Investment Strategies