Popular on EntSun
- Female Motorsports Sponsorship & Expansion; Acquisition Agreement of UAE-Based Sports Incubator by Online Lottery & Sports Game Provider: Lottery.com - 224
- Experience Trembling Firsthand with the New AgeMan® Tremor Simulator - 205
- WOA Entertainment Group Unveils Independent No.1's: Breakthrough Artists II —A Celebration of Indie Excellence - 205
- Edtech Startup Young Commanders Launches 'Visionaries Without Sight' Collection Celebrating Blind and Visually Impaired Historical Figures - 197
- Introducing Nene Wear: The Bold, Sporty Fashion Brand by Jeanne Myrick, Powered by KLM Enterprises - 184
- Let's Talk Series: At the Crossroads: Immigration Today - 145
- TEDxInglewood Returns to The Miracle Theater on August 9, 2025 — Celebrating Bold Ideas and Community Energy - 128
- IRF Builders Forum Brings Global Leaders to Washington, D.C. to Advance Religious Freedom Through Cooperative Engagement - 122
- Manhattan Boutique Real Estate Collaborates with InterContinental Hotels NYC in a Branding Partners Personalized Approach - 119
- Token-Operated Sake Service Opens at Tobu Nikko Station - 117
Similar on EntSun
- Plan to Launch Silo Technologies' Cybersecurity Pilot Program for Ultimate Nationwide Deployment via Exclusive Partnership: Stock Symbol: BULT
- AI-Based Neurotoxin Countermeasure Initiative Launched to Address Emerging National Security Needs: Renovaro, Inc. (N A S D A Q: RENB)
- $796,000 in Q2 Revenue Marks Highest Earnings to Date on 3 Trailing Quarters of Profitability in Multi-Billion Homebuilding Sector: Stock Symbol: IVDN
- Cybersecurity is THE Hot Market Sector; Revenues, Earnings & Profit matter; Only 33 Million Shares + a Huge Short Position Equal an Undervalued Stock
- Despite Global Calls for a Ban, US Child Psychiatry Pushes Electroshock for Kids
- Preliminary.online Introduces Short-Term Job-Readiness Courses with Employer-Verified Certifications
- Agreement to Supply US-Based Defense Provider with Thin-Film Solar Tech for Orbital Application; Ascent Solar Technologies, Inc. (N A S D A Q: ASTI)
- Suzanne Harp named Managing Director in Texas, USA
- $10 Million Acquisition of GXR World Sports Assets Energizes Global Launch of Sports.com Super App by Online Lottery-Sports Game Provider: Lottery.com
- Investor Spotlight: Cycurion, Inc. (N A S D A Q: CYCU) Secures $69M in Contracts Amid Surging Demand for AI-Powered Cybersecurity Solutions
Plan Signed to Purchase Kadima Neuropsychiatry Institute as Clinical Treatment Model and Leading Investigative Site Addressing Suicidal Depression
EntSun News/11058910
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) $NRXP Set Up for $300 Million in Milestones on Tiered Double-Digit Royalties
MIAMI - EntSun -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to Global Market
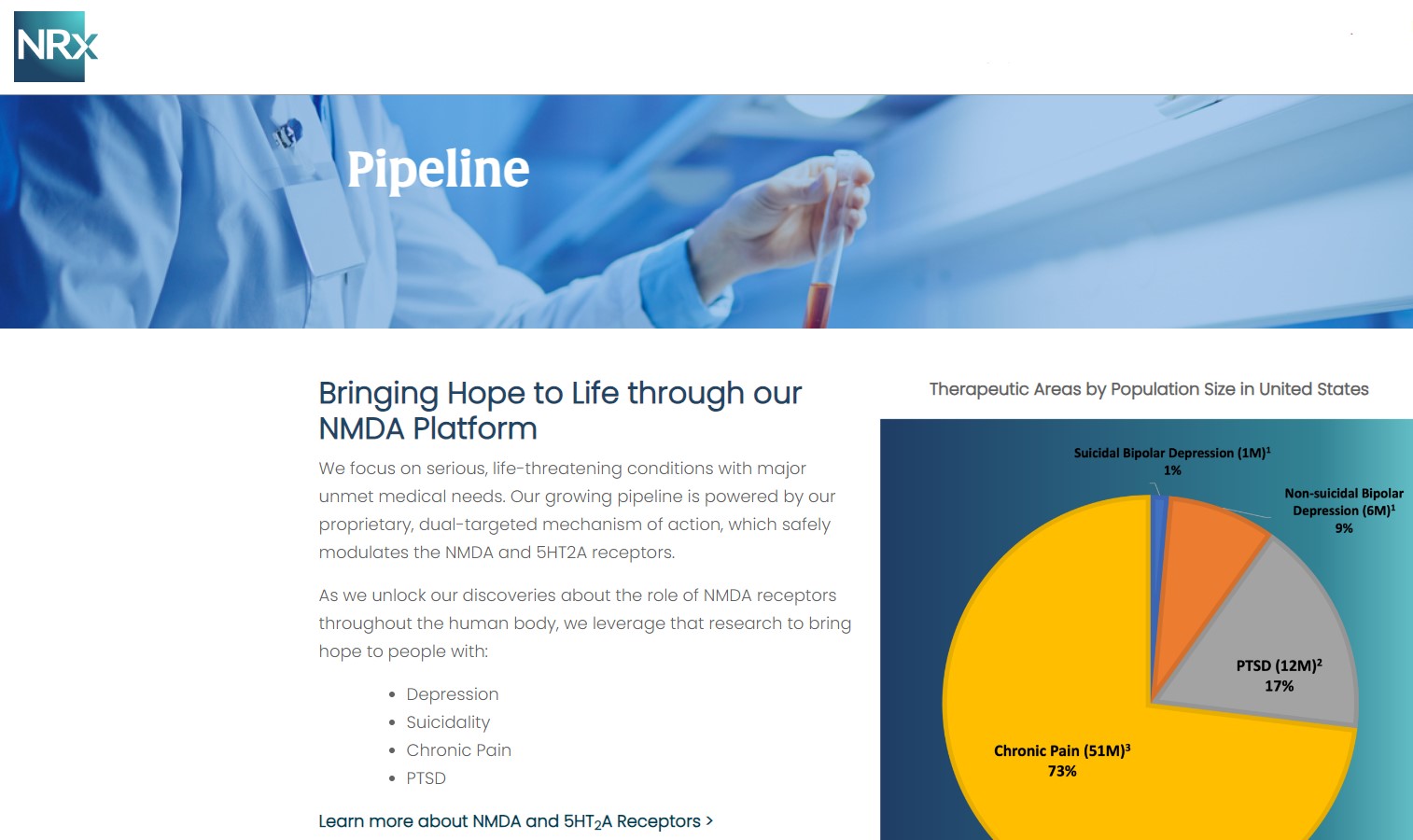
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on EntSun News
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, transcranial magnetic stimulation ("TMS") as well as medication management. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
More on EntSun News
"We are committed to delivering safer, more effective treatments for patients with suicidal depression," said Jonathan Javitt MD MPH, CEO of NRXP. "NRX-100 eliminates the need for benzethonium chloride, a compound with well-documented safety concerns, and reflects our belief that patients in crisis deserve therapies formulated with their long-term well-being in mind. With the recent FDA fee waiver now in place, we remain on track to complete our NDA submission this quarter — a critical step toward bringing this innovation to patients in need."
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
NRXP notes recent statements by the Secretary of Health and Human Services supporting the importance of psychedelic drugs to treat severe depression and PTSD. Ketamine is believed to have a beneficial effect through its role in blocking the NMDA receptor of the brain and causing increased levels of beneficial neurotransmitters in the brain, with resulting formation of new brain cell connections (synapses).
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. ((Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to Global Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on EntSun News
- The Naturist World Just Shifted — NaturismRE Ignites a Global Resurgence
- $796,000 in Q2 Revenue Marks Highest Earnings to Date on 3 Trailing Quarters of Profitability in Multi-Billion Homebuilding Sector: Stock Symbol: IVDN
- Cybersecurity is THE Hot Market Sector; Revenues, Earnings & Profit matter; Only 33 Million Shares + a Huge Short Position Equal an Undervalued Stock
- Despite Global Calls for a Ban, US Child Psychiatry Pushes Electroshock for Kids
- Franco Polished Plaster Celebrates 35 Years of Bringing Walls to Life in the UK
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, transcranial magnetic stimulation ("TMS") as well as medication management. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
More on EntSun News
- India's Home Textile Manufacturers See Rising Demand From Us Buyers, Enabled By rivexa
- Spartan & Guardians Partner with Guitar Legend Buckethead to Support Global Child Rescue Efforts
- Is the Entertainment Industry in Legal Tragedy: Was Nas's song The World is Yours taken literally
- Preliminary.online Introduces Short-Term Job-Readiness Courses with Employer-Verified Certifications
- Psychologist-Turned-Hermeticist Releases Modern Guide to the Seven Hermetic Principles
"We are committed to delivering safer, more effective treatments for patients with suicidal depression," said Jonathan Javitt MD MPH, CEO of NRXP. "NRX-100 eliminates the need for benzethonium chloride, a compound with well-documented safety concerns, and reflects our belief that patients in crisis deserve therapies formulated with their long-term well-being in mind. With the recent FDA fee waiver now in place, we remain on track to complete our NDA submission this quarter — a critical step toward bringing this innovation to patients in need."
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
NRXP notes recent statements by the Secretary of Health and Human Services supporting the importance of psychedelic drugs to treat severe depression and PTSD. Ketamine is believed to have a beneficial effect through its role in blocking the NMDA receptor of the brain and causing increased levels of beneficial neurotransmitters in the brain, with resulting formation of new brain cell connections (synapses).
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. ((Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on EntSun News
- Endoacustica Europe Unveils iPhone 13 Pro Max Spy Phone—Pure Hardware, Zero Software Changes
- Suzanne Harp named Managing Director in Texas, USA
- $10 Million Acquisition of GXR World Sports Assets Energizes Global Launch of Sports.com Super App by Online Lottery-Sports Game Provider: Lottery.com
- Shop American Made Goods: New Online Marketplace My American Goods Curates the Best of U.S. Made
- Investor Spotlight: Cycurion, Inc. (N A S D A Q: CYCU) Secures $69M in Contracts Amid Surging Demand for AI-Powered Cybersecurity Solutions
- $328 Million Global Stroke Rehab Market Opportunity Awaits AI Telehealth Leader Following Selection for NIH Funded Phase 3 Clinical Study: VSee Health
- Ascent Solar Technologies Enters Collaborative Agreement Notice with NASA to Advance Development of Thin-Film PV Power Beaming Capabilities: ASTI
- VoodooSoft Unveils SiriusLLM: The World's First ChatGPT-Like AI Malware Detection Engine
- This Ain't Press. This Is Pressure — Star Command by RansomXX is Out Now
- An Exclusive VIP Reception Honoring Vocal Prodigy Alliana Lili Yang's Remarkable Achievements and Magazine Cover Spotlight
- New Trend in Event Rentals: Luvies Bounce House Offers Instagram-Worthy Experiences
- Joyce Carol Oates Returns to Hard Case Crime With DOUBLE TROUBLE
- 2nd Annual (Neighbor) Hood Fest Announces Lineup and Sponsors
- New AI Academy Helps Therapists Embrace Tech Without Losing Their Humanity
- Vocs AI Launches Groundbreaking AI Music Label, Roster to Feature Over 40 Licensed AI Artists
- ELKLOOK Unveils Next-Gen Lens Tinting Innovation
- IQSTEL Surges Toward $400M Run Rate with $101.5M in Revenue—Reinforces Billion-Dollar Vision Backed by Fintech, AI, and Cybersecurity
- Alpha Modus Files 7th IP Action Against Rackspace Following $3M CEO Investment and Strategic Partnership Expansion
- Mortgage Rates And Demand Are Stuck In A Holding Pattern
- Coker Completes Acquisition of Healthcare Cost Solutions, a Leading Expert in Technology-Enabled Compliance Services