Popular on EntSun
- Guests Can Save 10 Percent Off New Vacation Rental Homes at KeysCaribbean's Village at Hawks Cay Villas - 127
- Kaplan Morrell Law Firm Represents Former NHL Player in Workers' Compensation Case Drawing National Attention - 121
- Metro Detroit teen Lola Winters turns viral TikTok fame into a sold-out clothing brand - 116
- UK Financial Ltd Celebrates Global Recognition as MayaCat (MCAT) Evolves Into SMCAT — The World's First Meme Coin Under ERC-3643 Compliance - 116
- America's Leading Annuity Expert Carlton Cap Averil II Joins Tom Hegna on "Financial Freedom with Tom Hegna" - 115
- Powering the Next Frontier of the $1 Trillion Space Economy: Ascent Solar Technologies (N A S D A Q: ASTI) - 107
- Epic Pictures Group Sets North American Release Date for the Action Thriller LOST HORIZON - 107
- Cut Costs & Boost Profits with the First Major Upgrade in 30 YEARS Replacing Rotary Lasers and Historic Clear Tube Altimeter Bubbles - 103
- U.S. Military to Benefit from Drone Tech Agreement with NovaSpark Energy, Plus Longer NASA Space Missions via Solar Power Leader: Ascent Solar $ASTI - 101
- Make This Fall Your Most Stylish Yet with Nickel-Free Bestsellers from Nickel Smart
Similar on EntSun
- Cummings Graduate Institute for Behavioral Health Studies Celebrates New DBH Graduates
- $80M+ Backlog as Florida Statewide Contract, Federal Wins, and Strategic Alliance Fuel Next Phase of AI-Driven Cybersecurity Growth: Cycurion $CYCU
- High-Conviction CNS Disruptor Aiming to Transform Suicidal Depression, Ketamine Therapeutics, and TMS - Reaching Millions by 2030
- Talagat Business Academy Announces Joint Certificate Program With The University of Chicago Booth School of Business
- Slotozilla Launches New Report on How AI Is Reshaping Careers and Society
- OKAVA Pharmaceuticals Announces First Cat Dosed in MEOW-1 Study of OKV-119, the World's First Clinical-Stage GLP-1 Weight-Loss Therapy for Pets
- Explosive Growth in U.S. Cryptocurrency Cloud Mining Sets The Stage for New Platform Launch with Daily Rewards in a Transparent Revenue-Share Model
- Qtex Cierra Ronda de $7 Millones para Estandarizar la Banca Transfronteriza en los Mercados Emergentes de Latinoamérica
- FDA Accepts ANDA for KETAFREE™ as Analyst Sets $34 Price Target for NRx Pharmaceuticals: (N A S D A Q : NRXP) NRx is Poised for a massive Breakthrough
- Broadway Smile Boutique Unveils Modern Website for Enhanced Patient Experience
$750 Million Market Projected to Reach $3.35 Billion; Huge Opportunity for Superior Preservative-Free Ketamine Drug Treating Suicidal Depression $NRXP
EntSun News/11073068
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Refiles Abbreviated New Drug Application; $40 Price Target in New H. C. Wainright Research Report
MIAMI - EntSun -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
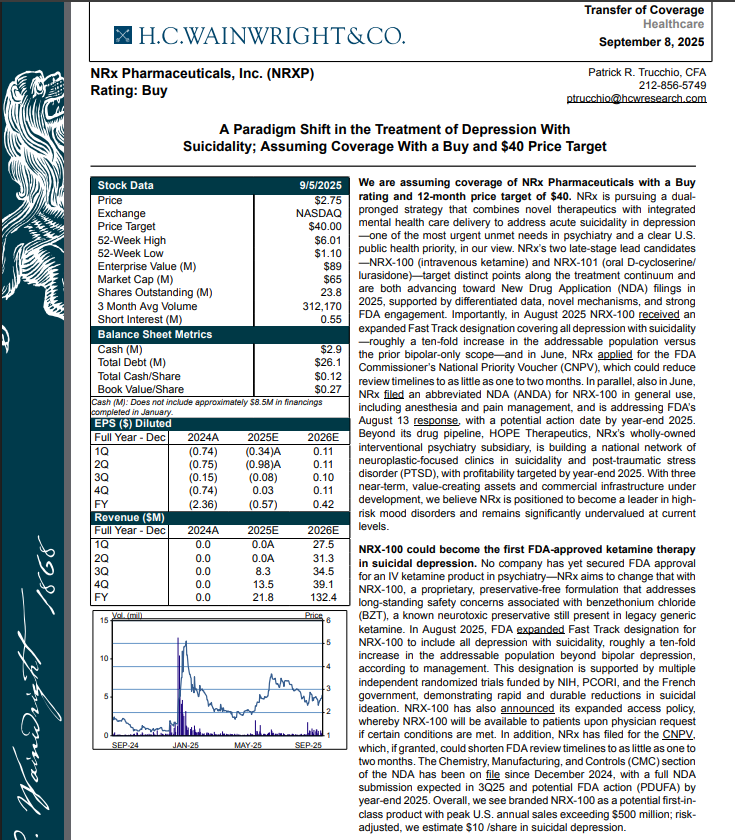
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Re-Filing of Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
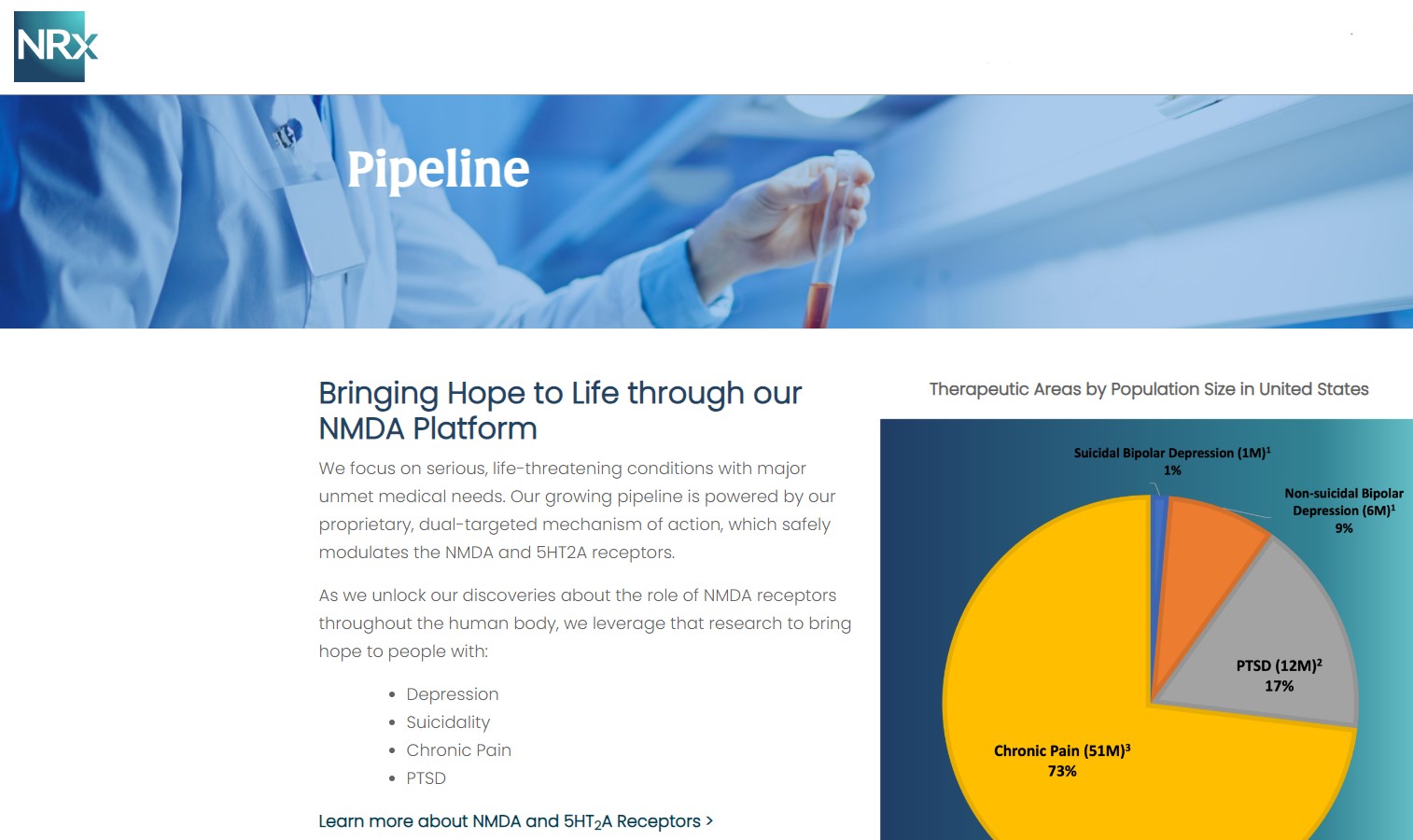
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on EntSun News
NRXP Re-Files Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine
On September 29th NRXP announced the re-filing of its Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration (FDA) for KETAFREE™, its preservative-free IV ketamine formulation, for use in all existing approved indications. The filing follows FDA grant of approval of its Suitability Petition for the NRXP proposed strength of preservative-free ketamine.
The current annual ketamine market is estimated at $750 million, with global demand for ketamine projected to grow to $3.35 billion by 2034. This does not include the widespread use of compounded ketamine by clinics unable to obtain manufactured drug. NRx aims to capture a significant share of the current market. According to a 2021 survey, an estimated 5.1 million Americans had received ketamine for medical uses in their lifetime3, a number that continues to grow with increased clinical focus on this important medication. Ketamine currently faces a severe drug shortage according to the American Society of Hospital Pharmacists. Accordingly, NRXP is seeking priority review from FDA.
NRXP previously filed a citizen's petition with the FDA to remove benzethonium chloride (BZT), a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. The FDA has previously disallowed the use of BZT in hand cleansers and topical antiseptics. A related preservative, benzalkonium chloride has demonstrated corneal and conjunctival toxicity in artificial tears and glaucoma medications, leading to use of preservative-free alternatives. NRXP has filed expert testimony from accredited toxicologists regarding the toxicity of BZT, which is not generally recognized as safe (GRAS) by the FDA. Removal of potentially harmful preservatives from foods is a stated priority in the MAHA report and HHS leadership has additionally targeted preservatives in vaccines. BZT was originally added to ketamine when it was first formulated in the 1970s to maintain stability and sterility using the container closure systems then available. NRx has demonstrated long term stability and sterility with a patented preservative-free formulation using modern manufacturing methods.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
More on EntSun News
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Re-Filing of Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on EntSun News
- Cummings Graduate Institute for Behavioral Health Studies Celebrates New DBH Graduates
- $80M+ Backlog as Florida Statewide Contract, Federal Wins, and Strategic Alliance Fuel Next Phase of AI-Driven Cybersecurity Growth: Cycurion $CYCU
- High-Conviction CNS Disruptor Aiming to Transform Suicidal Depression, Ketamine Therapeutics, and TMS - Reaching Millions by 2030
- Top10Christmas.co.uk Releases the UK Christmas Toy Trends 2025 Report
- Talagat Business Academy Announces Joint Certificate Program With The University of Chicago Booth School of Business
NRXP Re-Files Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine
On September 29th NRXP announced the re-filing of its Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration (FDA) for KETAFREE™, its preservative-free IV ketamine formulation, for use in all existing approved indications. The filing follows FDA grant of approval of its Suitability Petition for the NRXP proposed strength of preservative-free ketamine.
The current annual ketamine market is estimated at $750 million, with global demand for ketamine projected to grow to $3.35 billion by 2034. This does not include the widespread use of compounded ketamine by clinics unable to obtain manufactured drug. NRx aims to capture a significant share of the current market. According to a 2021 survey, an estimated 5.1 million Americans had received ketamine for medical uses in their lifetime3, a number that continues to grow with increased clinical focus on this important medication. Ketamine currently faces a severe drug shortage according to the American Society of Hospital Pharmacists. Accordingly, NRXP is seeking priority review from FDA.
NRXP previously filed a citizen's petition with the FDA to remove benzethonium chloride (BZT), a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. The FDA has previously disallowed the use of BZT in hand cleansers and topical antiseptics. A related preservative, benzalkonium chloride has demonstrated corneal and conjunctival toxicity in artificial tears and glaucoma medications, leading to use of preservative-free alternatives. NRXP has filed expert testimony from accredited toxicologists regarding the toxicity of BZT, which is not generally recognized as safe (GRAS) by the FDA. Removal of potentially harmful preservatives from foods is a stated priority in the MAHA report and HHS leadership has additionally targeted preservatives in vaccines. BZT was originally added to ketamine when it was first formulated in the 1970s to maintain stability and sterility using the container closure systems then available. NRx has demonstrated long term stability and sterility with a patented preservative-free formulation using modern manufacturing methods.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
More on EntSun News
- LocaXion and Asseco CEIT Announce First-to-Market RTLS-Driven Digital Twin Platform for Healthcare, Manufacturing, and Logistics
- Slotozilla Launches New Report on How AI Is Reshaping Careers and Society
- Really Cool Music Announces That Its Debut Single "I Move In Silence" Has Been Streamed Around the World
- High Rise: Path to Nowhere (2022) — A High-Stakes Thriller Filmed in Charlotte, North Carolina
- High Rise: Path to Nowhere (2022) A High-Stakes Thriller That Will Keep You On the Edge of Your Seat
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on EntSun News
- Universal Championship Wrestling Presents: Universal Fury 17 – The Christmas Special
- New Daily Oracle Neurology Online A Comprehensive Spiritual Guidance Platform
- Confidence Apparel Takes Over H&M Michigan Avenue For Holiday Confidence Pop up
- Finland Unveils New B2B Operating Model: Mandatory Supplier Licensing and Veikkaus Split Confirmed for 2027
- 100% Bonus Depreciation Places New Spotlight on Off The Hook Yacht Sales Inc. (N Y S E: OTH) as a Major Player in the $57 Billion U.S. Marine Market
- CNCPW Benchmarks Global Industry Standards: Integrating SEC Compliance with 3 Million TPS Architecture for Institutional Infrastructure
- The Patina Collective & Artist Jesse Draxler Debut "The Machine of Loving Grace"
- Smile! Dental Center Named 2025 "Best Dentist" in North Pittsburgh, Celebrating High-Tech Care and Heartfelt Service
- Dr. Johnny Shanks, As Seen on TV, Announces 20% Off Dental Implant Treatments | Tennessee's Leading All-on-X Provider
- Star Sleep & Wellness Expands to Pearland, Texas — Bringing Life-Changing Sleep Care to More Communities
- Fort Lauderdale Dentist Dr. Taskonak & IN A DAY SMILE Receive Emmy Nomination for Life-Changing Documentary "The Weight of a Smile"
- Men's Health Network Highlights Major 2025 Achievements & Launches New Donation Platform For Greater Impact
- BET and Soul Train Awards - GONE! - Introducing The World Hip Hop Awards
- Nickelodeon "All That" Alumni Angelique Bates' 45th Birthday Benefit: All That & A Bag of Jokes
- Australian Aboriginal Cultural Immersions and First Nations Workshops
- FabFestivals Launches Spanish Version of Fast-Growing International Festival Directory
- Cleo Harper Black Friday Offer Sells Out Early
- New Climate Thriller Explores Corporate Control of Weather in "The Rainmakers"
- Uk Financial Ltd Featured In New York Business Now — 2019 Gold-Backed Bitcoin Prediction Now Becomes SEC Security Token Filing
- Thousands Are Rushing to Buy a $7 Ticket — But Most People Will Still Miss Their Shot at 327 Acres