Popular on EntSun
- Female Motorsports Sponsorship & Expansion; Acquisition Agreement of UAE-Based Sports Incubator by Online Lottery & Sports Game Provider: Lottery.com - 244
- IRF Builders Forum Brings Global Leaders to Washington, D.C. to Advance Religious Freedom Through Cooperative Engagement - 208
- BillBoards Inc. Hits the Road with God Bless America Tour and Reality Series Now Streaming on Tubi TV - 158
- Token-Operated Sake Service Opens at Tobu Nikko Station - 136
- Honoring Black History, Culture, and Community in Fall River - 133
- databahn Launches GenAI Sales Intelligence Platform to Revolutionize Fortune 500 and Global 2000 Account Research - 126
- Byrd Davis Alden & Henrichson Launches Independence Day Safe Ride Initiative with 500 Free Uber Credits - 119
- Brandi Little to Debut Powerful Soul Single "Thought It Was Love" June 27 on MoonTownTunes.com - 113
- The ITeam Ranked on Channel Partners 2025 MSP 501—Tech Industry's Most Prestigious List of Managed Service Providers Worldwide - 112
- Agreement to Supply US-Based Defense Provider with Thin-Film Solar Tech for Orbital Application; Ascent Solar Technologies, Inc. (N A S D A Q: ASTI) - 112
Similar on EntSun
- The Blue Luna Encourages Local Schools to Take Steps to Enhance Safety for Students and Staff
- Smart Resnse Unveils Smart Resnse(SRMS) Token-Powered AI Orchestration Platform to Revolutionize Multi-Billion Dollar Market
- Josh and Heidi Follow Up the Much Anticipated and Successful Launch of the "Spreading the Good BUZZ" Podcast with a Personal Request
- Revolutionary Blockchain Platform Okh Finance Announces Okh Finance(OKKH) Token Launch to Transform Global Asset Leasing Market
- Stuck Doing Math or Figuring Out Life's Numbers? Calculator.now Makes It Stupidly Simple
- The World's Largest Green Economic Revolution Emerges as Nature, Tech, and Finance Converge
- Vinnetwork Unveils Decentralized AI Platform with Vinnetwork(VIN) Token to Challenge Tech Giants' Data Monopoly
- Pyro Marketing Opens New Digital Marketing Company to Power Growth for Fitness and Ecommerce Brands
- Dr. John Salerno of Salerno Wellness Introduces Their New Full Body Capsule for Advanced LED Light Therapy Patient Treatments
- $14M Expansion Deal with Famed David Lloyd Highlights Rebrand of Sports, Entertainment and Gaming Innovation by AI Driven, Online Fan Engagement Co
$4.3 Million Waiver Exemption Granted by FDA on New Drug Application Fee for Treatment Addressing Suicidal Depression, PTSD: NRx: Stock Symbol: NRXP
EntSun News/11057615
$300 Million Set up in Milestones for $NRXP on Tiered Double-Digit Royalties
MIAMI - EntSun -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
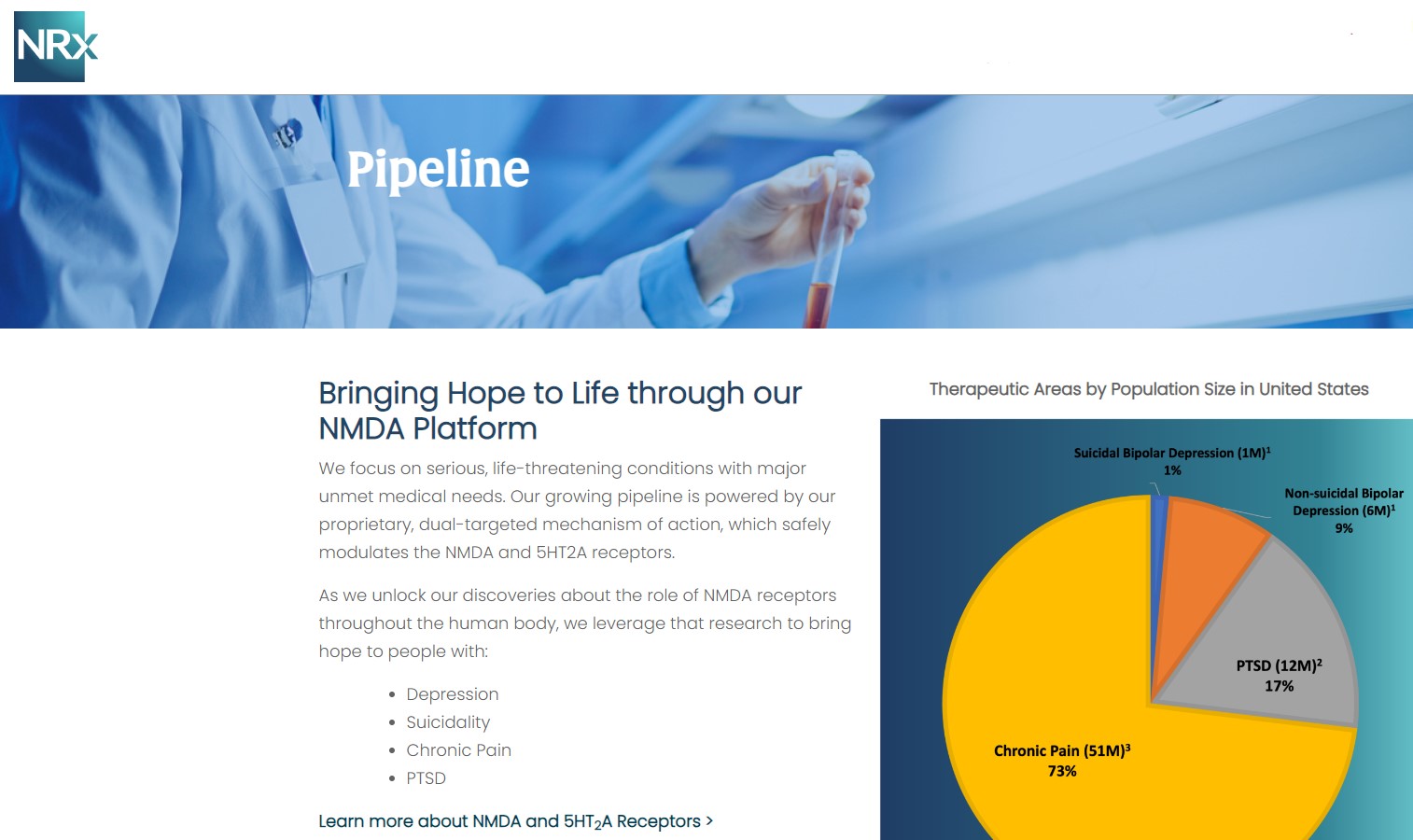
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on EntSun News
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
NRXP notes recent statements by the Secretary of Health and Human Services supporting the importance of psychedelic drugs to treat severe depression and PTSD. Ketamine is believed to have a beneficial effect through its role in blocking the NMDA receptor of the brain and causing increased levels of beneficial neurotransmitters in the brain, with resulting formation of new brain cell connections (synapses).
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
More on EntSun News
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics
On March 24th NRXP announced signing of a binding Letter of Intent to acquire a majority interest in Neurospa TMS Holdings, LLC. Neurospa operates six interventional psychiatry clinics on Florida's Gulf Coast and will constitute a key element of HOPE's Florida network going forward. Neurospa is revenue generating and EBITDA positive.
Neurospa leverages state-of-the-art interventional psychiatry procedures, including Ketamine Infusion Therapy, Transcranial Magnetic Stimulation (TMS), and Spravato®, augmented by traditional psychiatry and talk therapy to provide a full continuum of care for people with depression, suicidality, PTSD, anxiety, and related disorders. TMS is an FDA-approved procedure in which focused electromagnetic treatment has been demonstrated to reduce symptoms of depression. Ketamine and Spravato® are similarly known to reduce symptoms of depression and both forms of treatment are increasingly used in an additive manner.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on EntSun News
- Smart Resnse Unveils Smart Resnse(SRMS) Token-Powered AI Orchestration Platform to Revolutionize Multi-Billion Dollar Market
- Josh and Heidi Follow Up the Much Anticipated and Successful Launch of the "Spreading the Good BUZZ" Podcast with a Personal Request
- The Battle for the Enchanted Forest Brings Fantasy, Fun, and Fundraising!
- Revolutionary Blockchain Platform Okh Finance Announces Okh Finance(OKKH) Token Launch to Transform Global Asset Leasing Market
- Cover Girl Finalist Teisha Mechetti Questions Legitimacy of Inked Originals Competition, Demands Transparency
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
NRXP notes recent statements by the Secretary of Health and Human Services supporting the importance of psychedelic drugs to treat severe depression and PTSD. Ketamine is believed to have a beneficial effect through its role in blocking the NMDA receptor of the brain and causing increased levels of beneficial neurotransmitters in the brain, with resulting formation of new brain cell connections (synapses).
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
More on EntSun News
- Easton & Easton, LLP Files Suit Against The Dwelling Place Anaheim & Vineyard USA Over Abuse Allegations
- AI Visibility: The Key to Beating Google's AI Overviews and Regaining Traffic
- Stuck Doing Math or Figuring Out Life's Numbers? Calculator.now Makes It Stupidly Simple
- Colbert Packaging Announces WBENC Recognition
- DivX Empowers Media Enthusiasts with Free Expert Guides for Advanced MP4 Management
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
Binding Letter of Intent with Neurospa TMS Holdings, LLC for Expansion of its Planned International Network of Interventional Psychiatry Clinics
On March 24th NRXP announced signing of a binding Letter of Intent to acquire a majority interest in Neurospa TMS Holdings, LLC. Neurospa operates six interventional psychiatry clinics on Florida's Gulf Coast and will constitute a key element of HOPE's Florida network going forward. Neurospa is revenue generating and EBITDA positive.
Neurospa leverages state-of-the-art interventional psychiatry procedures, including Ketamine Infusion Therapy, Transcranial Magnetic Stimulation (TMS), and Spravato®, augmented by traditional psychiatry and talk therapy to provide a full continuum of care for people with depression, suicidality, PTSD, anxiety, and related disorders. TMS is an FDA-approved procedure in which focused electromagnetic treatment has been demonstrated to reduce symptoms of depression. Ketamine and Spravato® are similarly known to reduce symptoms of depression and both forms of treatment are increasingly used in an additive manner.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on EntSun News
- John Duffy Hosts Sister Irene O' Neill, Founder and President, and Kelly Mallon Young
- Heritage at South Brunswick Offers Immediate Townhome Appointments and Special Mortgage Incentive Fast-Moving Sales
- American Made Ivey Abitz Bespoke Clothing Travels for Summer
- NASA Collaborative Agreement for Supply of Thin-Film Solar Tech for Orbital Application to Advance Development of Thin-Film PV Power Beaming: $ASTI
- Exciting New Era of Sports, Entertainment & Gaming Innovation Spotlighted by Rebrand of Expanding AI Driven, Online Fan Engagement Company: SEGG Media
- Service Ninjas Debuts First-of-Its-Kind "Membership" Platform for Home Service Pros
- Bruce In The USA Brings Immersive Bruce Springsteen Celebration to Frederick This October
- BIYA Forecasts 2025 Surge with ¥300M ($41.8 M USD) in Revenue and ¥25M Profit from Cloud Based HR Solutions: Baiya Intl. Group (N A S D A Q: BIYA)
- Paul E. Saperstein Co. Announces Geographic Expansion of Auction Services
- Florida Broker Bent Danholm Featured in the Daily Mail's U.S. Real Estate Coverage
- Robin Launches Legal Intelligence Platform to solve intelligence gap in Fortune 500 legal teams
- Melissa B. Releases Digitally Independent: Empowering Music Artists with AI and Brand Strategy
- Mental: Elderly Abuse a Documentary
- Consumer Accountability Alliance Issues Formal Notice Alleging Proximate Liability for Medical Harm
- Rising to New Heights in the Mile High City with Destination: Scientology, Denver
- The City's Largest and Most Elegant Dinner Party Returns on September 13, 2025
- Utah Metal Fabricator Titan Forge Builds Momentum with Custom Steel Projects and Spiral Staircases
- Jason Koch: Pioneering the Future of Real Estate Development in New Jersey
- Voting Opens Tonight for the 2025 Spin Awards Honoring Excellence in Christian Gospel Radio
- Introducing LK Blue: The Cool-Girl Denim Brand That's Redefining LA Style Launches E-Commerce